Supplements
People often ask about supplements, but seldom are my replies satisfying.
In my opinion, they do not effectively treat disease, nor do they correct any obvious deficiencies in a visible way.
Nevertheless, we live in a situation in which the nutrient value of food is diminished. Our bodies evolved close to the land, eating fresh off the ground and the vine. Now we depend on harvesting, preservation, transportation, and marketing. The likelihood of receiving full nutrients from food is low. Even organic foods are transported and stored prior to consumption.
It is known that soil everywhere in North America is deficient in Zinc, and often in Selenium (though it is toxically high in the San Joaquin Valley). Vitamin C has been observed to be deficient even in orchards as a result.
Hence, my belief is that vitamins ought to be part of everyone’s diet unless there are allergies to the additives. Dramatic changes in energy or symptoms are not expected. Supplements are taken more as a kind of insurance policy against subtle deficiencies.
Undoubtedly dosages needed vary from person to person. Since there is no way to measure these distances, however, we can only go on generalizations. Here are those I have come up with from my reading and borrowed from others:
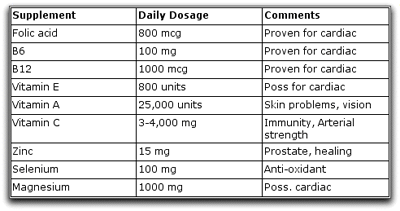
The scandal involved with supplements is that there is rampant cheating by producers of the products. There are no governmental regulations requiring that tablets or capsules in the food supplement realm contain what is described on the label!
In response, independent laboratories have been doing chemical analyses off the shelves and publishing results. As a general rule: about 25 % of the time, the tablet does not contain any of the substance on the label. Another 25% or more of the time, it may contain half the amount. Unfortunately, these statistics seem to apply to all companies found in health food stores at one time or another. For further information, look up the consumer laboratory websites. One such is www.consumerlab.com.
A recent development is that the FDA will offer a certification procedure based on some of its standard protocols for drugs. This agency will come into effect by Aug 30, as of last report. It will be purely voluntary and will examine companies who volunteer for purity, accuracy, safety, and labelling. To date, I know of only one company who has even applied for this certification, much less received it . It is called Nature Made, and I have seen it sold only in Longs Drugs thus far. I am hoping their willingness to be credentialed reflects their reliability.

